FATS, OILS, AND DISEASE
Mary Enig, PhD, who for many years has researched fats and oils at the University of Maryland, spoke to NOHA on March 26th. She advised us to avoid the hydrogenated fats that have proliferated in our processed foods. Stay with the simple foods that our ancestors have thrived on.
Producers want the fats in crackers, cookies, pastries, cakes, snack chips, imitation cheese, and candies to have a melting point above room temperature so that their product is not oily. Now they do not have to use lard, suet, chicken fat, or butter, as they did in the past. Producers achieve this change in melting temperature, by transforming the shape of some fatty acid chains from curved to straight. This allows them to use partially hydrogenated oils, such as soybean, cotton seed, peanut, or corn.
[Food industry] . . . want the fats in crackers, cookies, pastries, cakes, snack chips, imitation cheese, and candies to have a melting point above room temperature so that their product is not oily. . . . [They] . . . achieve this . . . by transforming the shape of some fatty acid chains from curved to straight. This allows them to use [much cheaper] partially hydrogenated oils, such as soybean, cotton seed, peanut, or corn.
In order for us to understand what processing has done to fats and oils, Dr. Enig gave us information on their composition. Summarizing a few essential highlights: all fats and oils contain fatty acids that have long chains of carbon atoms with hydrogen atoms attached. Unsaturated fatty acids contain fewer than the maximum possible number of hydrogen atoms. Almost always in nature a missing pair of hydrogen atoms leaves spaces on the same side of the carbon chain so that the chain is bent (the "cis" form).
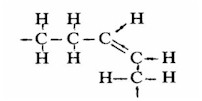
Part of a cis unsaturated chain of carbon (C) atoms, showing the curve where there are missing hydrogen (H) atoms.
Since the molecules with bent chains do not fit against each other compactly, unsaturated fats are liquid at body temperature rather than solid. Even when saturated, short-chain fatty acids give a softer consistency than do long-chain ones. Since the fatty-acid chains in seed oils are longer than those in butter, margarine with its straightened fatty-acid chains has a few more calories per pound than does butter, "but most people have been led to believe the opposite."
Trans fatty acids enter our bodies, are incorporated into our membranes, and have been shown to have many adverse effects. However, none of the large government studies have included any information on our consumption of trans fatty acids in their data banks.
The various fatty acids are found throughout our bodies<197>in membranes within and around our cells, in our circulatory systems, and in the linings of our organs. The various membranes must have the right consistency in order to function properly so both the shape and the length of fatty acid chains are extremely important.
Partial hydrogenation
The form of the carbon chains in the fatty acids is changed by hydrogenation -- a high temperature process using chemical catalysts and adding hydrogen. Complete hydrogenation would add the maximum number of hydrogen atoms and convert any oil into a completely saturated fat. Actually, partial hydrogenation is the customary and desired process because the remaining unsaturated carbon chains can be changed, often randomly moving the missing-hydrogen positions and converting them to the "trans " form that gives a melting point way above room temperature. In trans fatty acids a missing pair of hydrogen atoms leaves spaces on opposite sides of the carbon chain so that the shape becomes practically straight, like a saturated chain:
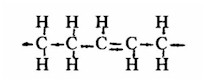
Part of a trans unsaturated chain of carbon (C) atoms, showing the straight section where there are missing hydrogen (H) atoms
In fact, Dr. Enig and her colleagues speak of the trans fatty acid proportions in products as "saturate equivalents." She showed us an interesting picture, drawn here, of a solid block and a bottle of oil:
|
"under hot lights" |
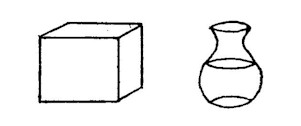
|
|
Partially
Hydrogenated Palm Oil 28% saturated
fat 45% saturated fat |
Anyone reading these percentages on a label would think that the fat in the product containing palm oil was much more saturated. However, the solid block on the left contains about 50 per cent trans fatty acids so it has well over 70 per cent in saturate equivalents. That solid block is often the base stock for margarine.
Food examples
Unhydrogenated foods can have tiny amounts of trans fatty acids. On the other hand, hydrogenated products often have high amounts. For example, real cheese contains less than one per cent, whereas imitation cheese contains 37 per cent trans fatty acids. Formerly, doughnuts were fried in old-fashioned lard containing no trans fatty acids; now the fat has about 35 per cent. French fries were formerly cooked in beef tallow with about 4 per cent trans fatty acids; now the hydrogenated soybean oil for cooking French fries has 42 per cent. Shortening with 42 per cent trans fatty acids is popular for making crackers and cookies, including Gerber's cookies for babies.
Diseases
Trans fatty acids enter our bodies, are incorporated into our membranes, and have been shown to have many adverse effects. However, none of the large government studies have included any information on our consumption of trans fatty acids in their data banks. Quoting from the handout supplied to NOHA by Dr. Enig:
This means that we really don't know what kind of fatty acid patterns people are consuming, so we certainly don't know whether the people with certain adverse health patterns consume more or less of any given class of fatty acids than those people without the adverse health patterns. All the rhetoric not-with-standing, we do not know if eating 50 per cent of our fat as saturated fatty acids (this is the percentage that is found in human milk) causes any health problem or relieves any health problem because we have never measured the dietary intake of the different fatty acid classes accurately!
In experiments trans fatty acids have been shown to have a number of adverse health effects. Here are just two examples: They interfere with certain enzymes that are needed to detoxify insecticides and they make some enzyme systems that potentiate carcinogens more active. Thinking of these health effects, Dr. Enig mentioned broccoli, which we are told can help protect us against cancer. However, if we cook it in margarine from some kind of partially hydrogenated oil with its trans fatty acids and if the broccoli was pesticided, then it is an open question whether or not we receive any protection.
Article from NOHA NEWS, Vol. XVII, No. 3, Summer 1992, pages 3-4.